Connections with Consequences
Early this year, the Director of the National Institute for Neurological Disorders and Stroke (NINDS) reflected upon its highlights of 2024. His post leads with a striking image of the full adult fly “connectome” published by a multi-institutional team, the FlyWire Consortium, last year:
Snippet of Figure 1 from Neuronal wiring diagram of an adult brain | Nature, licensed under CC-BY. Representation of the computed segmentation of all neurons and ‘wires’ (axons and dendrites) for a whole adult fly brain.
The acronym Ansyme begins with ‘advanced’ neuroscience and we should begin by saying the obvious. The FlyWire work – mapping out all the (microscopic) neurons and the (more microscopic) connections between them in a brain – clearly hits the ‘advanced’ mark! This landmark dataset was funded by the BRAIN Initiative, which is co-led by NINDS and whose own director likewise featured it as a 2024 highlight. The “A” in BRAIN also stands for advanced, and the Initiative was launched with an initial focus on pushing technological boundaries, i.e., what neuroscience can investigate. FlyWire demonstrates that fully.
Ansyme seeks to foster ‘systemic medicine’ applications of neuroscience, namely for humans, and someone outside this BRAINy club might wonder how relevant a highly detailed anatomical map of the fly brain may be to such ends. As if on cue, a mammalian connectome was just revealed last month from another multi-institutional team called MICrONS. This one was yet more technologically advanced, spanning a cubic millimeter of mouse visual cortex, and can be considered the crowning achievement in connectomics for the BRAIN Initiative’s first decade. For comparison, the adult fly brain spans about a quarter of a millimeter along its longest dimension.
While the fly connectome may be smaller in volume, it stands out for its completeness. Our last post showed that achieving whole-brain completeness for another neuroscience technology realm, spatial transcriptomics, was statistically transformative. It allowed an unbiased counting of cell types across brain regions that produced a major discovery: much higher-than-anticipated cell type diversity in the lower brain. While ‘more cell types’ is an abstract statement, its potential has been made more concrete by our series of early posts highlighting research showing that specific cell types of the lower nervous system can be mapped to specific functions, some of which are medical symptoms and states.
Could the completeness of a connectome, fly or otherwise, give us more gains in the realm of systemic medicine? We believe the answer is “yes”, judging from a tandem publication to the FlyWire data release from a Princeton-led team whose set of simulations and statistical assessment, grounded by the fly connectome data, led to their concise title “The fly connectome reveals a path to the effectome”.
We’ve been exploring neural effects across our early posts, which all used the techniques of optogenetics and/or chemogenetics. These can artificially stimulate a specific molecularly-identified set of neurons, which serves to prove sufficiency: that a specific small set of neurons has a big effect, such as triggering fainting or controlling sepsis. Some studies went further, using genetic knockout technologies to establish necessity, i.e., abolish the function if a key connection to or from that small neural set is severed. The combination of necessity and sufficiency has strong implications in formal logic and mathematics. Here, more practically, it strongly suggests the neural circuits being identified are quite likely the specific circuits for the specific functions studied.
We’ve been extrapolating from our examples that there might be a broader list, a ‘dictionary’, of such one-to-one mappings between neural circuits and medical symptoms and unwanted states. We might call these “mal-effectoids”, i.e., specific individual, countable bad effects with respect to the humans experiencing them. As patient stakeholders, our extrapolation from a few single circuit studies is admittedly grounded in hope.
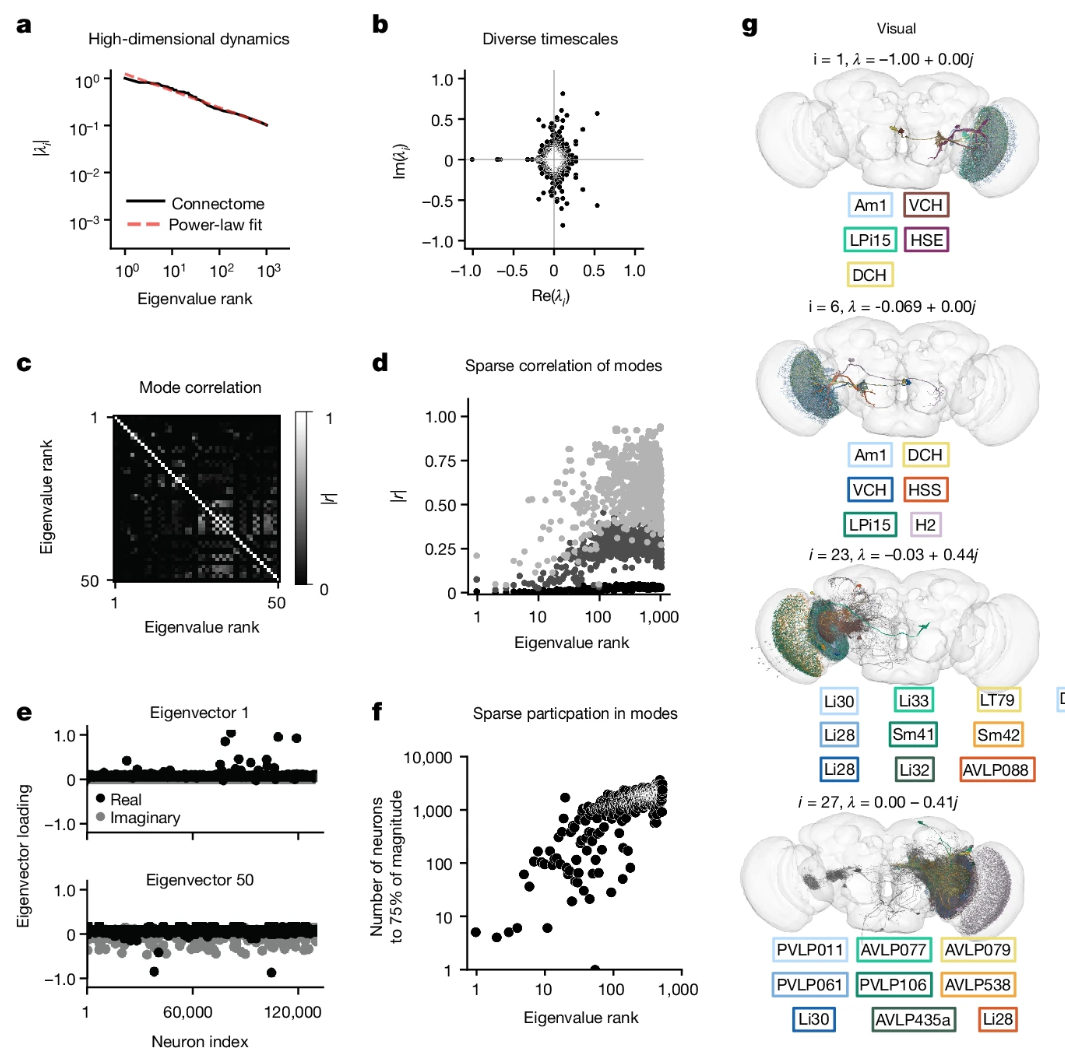
The Princeton-led analysis, on the other hand, was grounded in math. While the fly connectome itself was postmortem, and hence not a measurement of any physiological effects, it did reveal that connectivity was sparse – on average any neuron was only physically connected to ~.01% of other neurons – in addition to providing data regarding how strongly two neurons were connected (number of synapses) and the sign of that connection (predictable from the shape of those synapses). This information was formulated into a sparse matrix of over a billion entries whose properties could be studied with techniques of applied mathematics, seeking to identify which small sets of source neurons would have the largest global effects. The results were summarized in Figure 3 of the Princeton paper.
Snippet of Figure 3 from The fly connectome reveals a path to the effectome | Nature, licensed under CC-BY. Paper’s conclusion was largely based on a mathematical eigenvalue decomposition of the connectome matrix, whose eigenvalues and eigenvectors (summarized at left) correspond to putative ‘eigencircuits’ illustrated at right. The large number of roughly equal-valued eigenvalues and the low neuron counts of and correlation between these eigencircuits support the paper’s conclusions per the authors.
Their team conducted random stimulations of select neurons in the digital model and observed their effects over time, reaching the conclusion that “fly whole-brain dynamics are generated by a large collection of small circuits that operate largely independently of each other”. Their digital model was achieved by formulating the sparse connectome matrix as a linear dynamical operator, whose resulting eigenvalues and eigenfunctions reveal the dominant dynamics of the connectome ‘brought to life’ with random small stimuli.
Importantly, this large-scale virtual experiment could be grounded by real-world experimental data. Fly neuroscientists have been doing the same kinds of optogenetics experiments – one neural circuit at a time – as the rodent experiments we’ve highlighted in our early posts. This Princeton computational study “rediscovered” some of these known neural circuits, shown in their Figure 4, including the “ring attractor” circuits discovered to act like a compass for fly navigation.
The heart of their conclusion – “...a large collection of small circuits that operate largely independently of each other” – concisely connected several dots in my head. A “large collection” jibed with our guess there may be a broad ‘dictionary’ of effects, and hence mal-effects (aka symptoms), identifiable within our neural circuitry. That they “operate largely independently” jibed with my own experience with symptoms and dysfunctions seemingly having ‘minds of their own’ with somewhat predictable ‘logic’.
These ideas of piecemeal physiological logic went into my Idea submission during the spring 2024 public comment period for the ME/CFS Research Roadmap, which was also highlighted in the year-in-review post by the NINDS director. Public commentary on the scientific landscape appears to be expanding, e.g., a new philanthropy-backed “gap map” from Convergent Research that launched last month. Ansyme thus plans to make commentary on public scientific “maps” one of our activities, so those who plan and fund future scientific activities can see the connections between today’s advanced neuroscience and tomorrow’s systemic medicine.